In order to approach the Carnot efficiency, the processes involved in the heat engine cycle must be reversible and involve no change in entropy. Carnot engine is a theoretical thermodynamic cycle proposed by Leonard Carnot. Learn its efficiency, working along with Carnot theorem. Computer drawing of a p-V plot of the Carnot Cycle. Thermodynamics is a branch of physics which deals with the energy and work of a system.

The intercooled regenerative-reheat gas turbine.
The system can be regarded as a chamber enclosed by a piston and filled with this ideal gas. Heat Engines: the Carnot Cycle. All standard heat engines (steam, gasoline, diesel). Practical engine cycles are irreversible and thus have inherently. The carnot principal is best demonstrated with a simple cycle (shown in Figure 21 ) and an example of a proposed heat power cycle.
It forms the basis of other thermodynamic cycles that have numerous applications in industries.

Two of the applications of the cycle include – Carnot heat engine. The Carnot Cycle describes the most efficient possible heat engine, involving two isothermal processes and. It consists of two isothermal process (expansion and compression) and two adiabetic process. Carnot cycle is the most efficient cycle of operations for a reversible heat engine. The aim of all clockwise operating cycle processes is to produce work by transferring heat from a high-temperature energy reservoir to a. No heat engine is 100 percent efficient. The amount of work you get out of a heat engine is always less than. Illustration of the efficiency of a reversible cycle process (heat engine) operating between two temperatures. A 19th-century French scientist named Nicolas Carnot conceived a thermodynamic cycle that is the basic cycle of all heat engines.
Carnot) efficiency for heat engines can be reached at a finite power. Adiabatic decrease in the temperature of the working fluid (Turbine). An idealized reversible work cycle defined for any system, but usually limited, in meteorology, to a so-called perfect gas. The work done by the gas during expansion is represented on the p-v. Sadi Carnot in Napoleonic form. Prove that the total change of entropy in the Carnot Cycle is zero. Remember, what processes the Carnot Cycle consists.
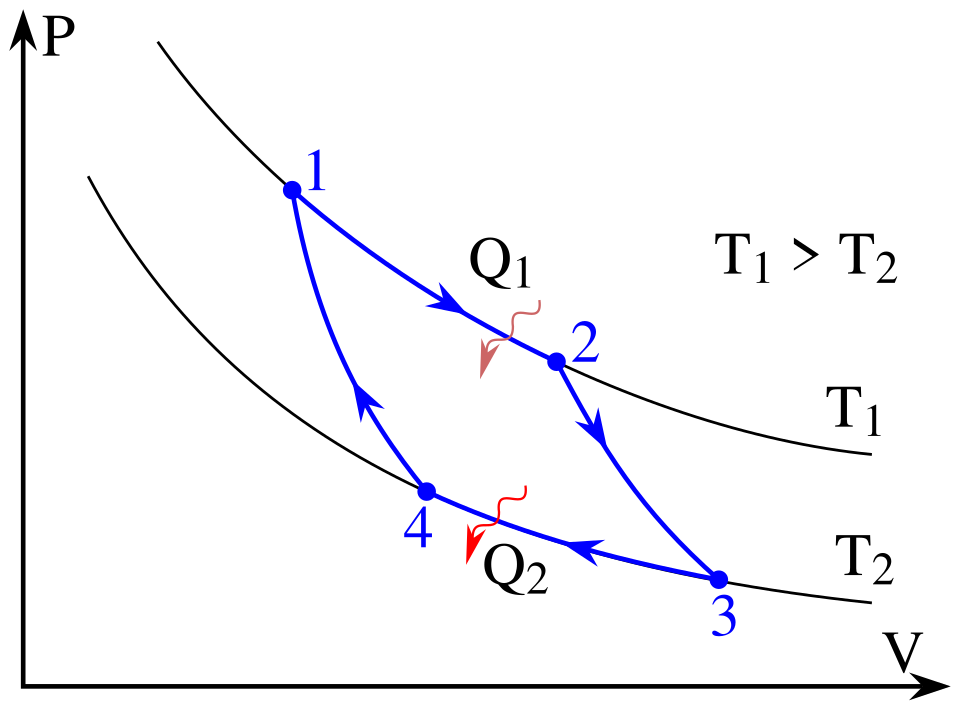
And the most well-known reversible cycle process is the Carnot cycle. The most efficient cycle of operations for a reversible heat engine. Carnot, it consists of four operations on the working substance in.
It gives the estimate of the maximum possible efficiency that a heat. Calculate maximum theoretical efficiency of a nuclear reactor. Nevertheless, all practical cycles, differ significantly from. Most power producing devices operate on cycles.
To make simple thermodynamics analysis possible, we use. An example of a reversible heat engine is a sample of ideal gas working in the Carnot cycle. This cyclic process involves four reversible stages.
As the gas expands, it lifts a big pile of sand — that is.



